Patents - Gilead Sciences Inc v NuCana Plc
Patents Court (Mr Justice Meade) Gilead Sciences Inc and another NuCana PLC [2023] EWHC 611 (Pat) (21 March 2023)
This was an action by Gilead Sciences Inc. and its British subsidiary Gilead Sciences Ltd ("Gilead") for revocation of two European Patents (UK) that had been granted to NuCana Plc ("NoCana"), namely EP 2955 190 B1 (“EP190”) and EP 3 904 365 B1 (“EP365”). NuCana complained that Gilead's product sofosbuvir infringed its patents. Mr Justice Meade, who tried the action, could see no real defence to the counterclaim if the claims of the patents were valid.
Grounds of Revocation
Gilead sought revocation of the patents on the following grounds:
- Added matter in that the Markush group definitions of the claims of the patents are not clearly and unambiguously disclosed in the original application;
- Lack of plausibility in that the Patents do not plausibly disclose any technical contribution common to substantially all the claimed compounds;
- Lack of industrial applicability;
- Lack of technical effect;
- Undue burden insufficiency; and
- The proposed amendments to EP190 were not allowable.
The Trial
The Invention
According to para 1 of the specification for EP190, the invention relates to nucleotide derivatives and the treatment of cancer. In the next paragraph, the specification refers to three examples including gemcitabine. it says that they are activated to their 5’ phosphate form but adds that the phosphate forms have bad membrane permeability, in response to which prodrugs had been developed.
Gemcitabine is a nucleoside analogue drug that has been successfully used in treating cancer, It is not easy to introduce that drug into cells The answer is to use prodrugs known as ProTides, They work by making phosphoramidate modifications to nucleoside analogues. The patents claim products defined by a Markush formula and cover ProTide nucleoside analogues as just described in which the nucleoside moiety is gemcitabine or gemcitabine-like.
More information on the invention appears between para [182] and para [210] of the judgment. The claims are discussed between para [211] and para [215].
The Issues
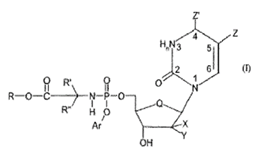
At para [8] the learned judge noted that Sofosbuvir fell within the above-mentioned Markush group:

Added Matter
Both patents had been granted on a divisional application from an application under the Patent Cooperation Treaty ("PCT"). It was agreed that the applications did not differ significantly from the PCT application. I stated above that Gilead complained that the Markush group definitions of the claims was not clearly and unambiguously disclosed in the original application. It alleged that NuCana had impermissibly cut down the Markush group by making deletions from the respective lists for the various substituent groups. which led to the disclosure of classes of compounds that had not been taught in the original applications. Those complaints applied both to the claims as they stood and also to the claims as they were proposed to be amended
In resolving Gilead's complaint Mr Justice Meade first considered the relevant law. He referred to the following passage from Lord Justice Kitchin's judgment in Nokia OYJ (Nokia Corporation) v IPCom GmbH & Co Kg [2012] EWCA Civ 567 (10 May 2012)om [2012] EWCA Civ 567 which quotes earlier judgments of the English courts and decisions of the European Patent Office Boards of Appeal:
“Added matter - the law
[46] The objection is founded upon Article 123 (2) EPC :
‘A European patent application or a European patent may not be amended in such a way that it contains subject matter which extends beyond the content of the application as filed.’
[47] The test for added matter was stated by Aldous J in Bonzel v Intervention (No 3) [1991] RPC 553 at 574 in these terms:
‘The decision as to whether there was an extension of disclosure must be made on a comparison of the two documents read through the eyes of a skilled addressee. The task of the Court is threefold:
(1) To ascertain through the eyes of the skilled addressee what is disclosed, both explicitly and implicitly in the application.
(2) To do the same in respect of the patent,
(3) To compare the two disclosures and decide whether any subject matter relevant to the invention has been added whether by deletion or addition. The comparison is strict in the sense that subject matter will be added unless such matter is clearly and unambiguously disclosed in the application either explicitly or implicitly.’
[48] In Case G 2/10, 30 August 2011, the Enlarged Board of the EPO explained in similar terms that an amendment can only be made 'within the limits of what the skilled person would derive directly and unambiguously, using common general knowledge, and seen objectively and relative to the date of filing, from the whole of the application as filed'.
[49] In Vector Corp v Glatt Air Techniques Ltd [2007] EWCA Civ 805, [2008] RPC 10, Jacob LJ elaborated aspects of the test to be applied and drew together various statements of principle from earlier cases at [4]-[9]:
[4] In Richardson-Vicks' Patent [1995] RPC 568 at 576 I summarised the rule in a single sentence:
‘I think the test of added matter is whether a skilled man would, upon looking at the amended specification, learn anything about the invention which he could not learn from the unamended specification.’
I went on to quote Aldous J in Bonzel. His formulation is helpful and has stood the test of time.
[5] The reason for the rule was explained by the Enlarged Board of Appeal of the EPO in G1/93 ADVANCED SEMICONDUCTOR PRODUCTS/Limiting feature [1995] EPOR 97 at [Reasons 9]:
'With regard to Article 123 (2) EPC, the underlying idea is clearly that an applicant shall not be allowed to improve his position by adding subject-matter not disclosed in the application as filed, which would give him an unwarranted advantage and could be damaging to the legal security of third parties relying upon the content of the original application.'
[6] Mr Richard Arnold Q.C. provided a clear articulation as to how the legal security of third parties would be affected if this were not the rule:
'The applicant or patentee could gain an unwarranted advantage in two ways if subject-matter could be added: first, he could circumvent the “first-to-file” rule, namely that the first person to apply to patent an invention is entitled to the resulting patent; and secondly, he could gain a different monopoly to that which the originally filed subject-matter justified.'
[7] Kitchin J has recently helpfully elaborated upon the Bonzel formulation in European Central Bank v Document Security Systems [2007] EWHC 600 (Pat), 26th March 2007:
'[97] A number of points emerge from this formulation which have a particular bearing on the present case and merit a little elaboration. First, it requires the court to construe both the original application and specification to determine what they disclose. For this purpose the claims form part of the disclosure (s. 130 (3) of the Act), though clearly not everything which falls within the scope of the claims is necessarily disclosed.
[98] Second, it is the court which must carry out the exercise and it must do so through the eyes of the skilled addressee. Such a person will approach the documents with the benefit of the common general knowledge.
[99] Third, the two disclosures must be compared to see whether any subject matter relevant to the invention has been added. This comparison is a strict one. Subject matter will be added unless it is clearly and unambiguously disclosed in the application as filed.
[100] Fourth, it is appropriate to consider what has been disclosed both expressly and implicitly. Thus the addition of a reference to that which the skilled person would take for granted does not matter: DSM NV's Patent [2001] RPC 25 at [195]-[202]. On the other hand, it is to be emphasised that this is not an obviousness test. A patentee is not permitted to add matter by amendment which would have been obvious to the skilled person from the application.
[101] Fifth, the issue is whether subject matter relevant to the invention has been added. In case G1/93, Advanced Semiconductor Products, the Enlarged Board of Appeal of the EPO stated (at paragraph [9] of its reasons) that the idea underlying Art. 123 (2) is that an applicant should not be allowed to improve his position by adding subject matter not disclosed in the application as filed, which would give him an unwarranted advantage and could be damaging to the legal security of third parties relying on the content of the original application. At paragraph [16] it explained that whether an added feature which limits the scope of protection is contrary to Art. 123 (2) must be determined from all the circumstances. If it provides a technical contribution to the subject matter of the claimed invention then it would give an unwarranted advantage to the patentee. If, on the other hand, the feature merely excludes protection for part of the subject matter of the claimed invention as covered by the application as filed, the adding of such a feature cannot reasonably be considered to give any unwarranted advantage to the applicant. Nor does it adversely affect the interests of third parties.
[102] Sixth, it is important to avoid hindsight. Care must be taken to consider the disclosure of the application through the eyes of a skilled person who has not seen the amended specification and consequently does not know what he is looking for. This is particularly important where the subject matter is said to be implicitly disclosed in the original specification.'
[8] When amendment of a granted patent is being considered, the comparison to be made is between the application for the patent, as opposed to the granted patent, and the proposed amendment (see the definition of ‘additional matter’ in s.76 (l) (b)). It follows that by and large the form of the granted patent itself does not come into the comparison. This case was to some extent overcomplicated by looking at the granted patent, particularly the granted claim 1.
[9] A particular, and sometimes subtle, form of extended subject matter (what our Act calls ‘additional matter’) is what goes by the jargon term ‘intermediate generalisation’. Pumfrey J described this in Palmaz's European Patents [1999] RPC 47, 71 as follows:
'If the specification discloses distinct sub-classes of the overall inventive concept, then it should be possible to amend down to one or other of those sub-classes, whether or not they are presented as inventively distinct in the specification before amendment. The difficulty comes when it is sought to take features which are only disclosed in a particular context and which are not disclosed as having any inventive significance and introduce them into the claim deprived of that context. This is a process sometimes called 'intermediate generalisation'.'"
The judge reflected on the rationale for the added matter rule as expounded by the Enlarged Board of Appeal and Richard Arnold QC. In response to a submission that selecting specific elements from a larger set was limiting the scope of a claim rather than enlarging it, his lordship said at [243]:
"An applicant might file an application with a very broad Markush group without knowing or having any idea which of the compounds covered worked. If the applicant were allowed later in prosecution freely to cut down the Markush group by reducing the options in multiple lists with later knowledge of which compounds did work, it could keep the original application’s date of filing for an invention which was in fact not disclosed in it (indeed one would say that the selection-type invention had not even been made at the time of filing). This would of course be unfair on a third party who had in fact, earlier than the applicant but after the original application, worked out which compounds worked."
He also said that "making a selection from multiple lists for which there is no basis, if permitted, could cut across the expectation of third parties who had concluded both that a broad class disclosed in an earlier application was invalid (for insufficiency or over the prior art), and also that there was no narrower class disclosed to which the applicant for the patent could fall back, or perhaps that any narrower fall back would not be infringed."
Mr Justice Meade referred to Mr Justice Arnold's review of the law on whether a selection from a wider set constitutes added matter between [288] and [293] of his judgment in Merck Sharp And Dohme Ltd v Shionogi & Co Lti [2016] EWHC 2989 (Pat) and his decision in Idenix Pharmaceutical, Inc v Gilead Sciences, Inc and others [2014] EWHC 3916 (Pat) which was upheld by the Court of Appeal. He also quoted para 1.6.3 of the 10th edition of the EPO Case Law Book. In para [283] the judge discerned the following principles:
"i) Do the deletions single out a particular combination of specific meanings, i.e. a hitherto not specifically mentioned individual compound or group of compounds?
ii) Or, do the deletions merely maintain the subject matter as a generic group of compounds differing only from the original group by its smaller size?
iii) It is relevant to consider whether the deletions 'generate another invention'. Another invention will be generated if the smaller group provides a technical contribution."
"i) Do the deletions single out a particular combination of specific meanings, i.e. a hitherto not specifically mentioned individual compound or group of compounds?
ii) Or, do the deletions merely maintain the subject matter as a generic group of compounds differing only from the original group by its smaller size?
iii) It is relevant to consider whether the deletions 'generate another invention'. Another invention will be generated if the smaller group provides a technical contribution."
Having considered the applicable law, the judge proceeded first to identify the disclosure of the PCT application that I mentioned above, next the disclosure of each of the patents in suit and finally to compare them in order to ascertain whether there was added matter within the meaning of art 123 (2) EPC.
Between [283] and [295] he set out the relevant disclosure of the PCT specification. Between [296] and [299] he considered the relevant disclosure of EP190 as proposed to be amended He noted at para [297]:
Between [283] and [295] he set out the relevant disclosure of the PCT specification. Between [296] and [299] he considered the relevant disclosure of EP190 as proposed to be amended He noted at para [297]:
"The statements of utility of the compounds disclosed remain textually the same as in the application but Gilead asserts that they are different in substance because they refer to different classes of compounds. For example paragraph [0059] refers to formula I as did the third paragraph on page 14 of the PCT, and formula I is to be amended."
In -paras [300] and [301] he made the comparison concluding at para [302] that there was plainly added matter. He set out his reasons as follows:
"[303] Compared with the PCT, the class of compounds of Formula I is much narrower. Y has to be F and X has to be F, Cl, Br or Me (except Cl is excised in the conditional amendment). By contrast the PCT allowed X and Y each to be any of H, F, Cl, Br, I, OH and Me.
[304] The new class is significantly different and the skilled person would not think that there had been a mere reduction still leaving the same generic class differing only in size.
[305] The problem is significantly exacerbated by the fact that the narrowing does not correspond to the statements of which possibilities are preferred: Y’s having to be F was one of the preferences expressed, but at the same time it was said that X ought also preferably to be F, H or OH, and the proposed amended claim keeps F but discards H and OH and replaces them with F, Cl, Br and Me, a mixture of those preferred and those not said to be preferred. There are also more detailed preferences for X and Y in the PCT depending on the base, but the amended claims do not correspond to them, either.
[306] When EP190 says that there are benefits to the compounds disclosed it is talking about a different class of compounds from those the PCT identified. There was no such claim for the class in the PCT.."
"[303] Compared with the PCT, the class of compounds of Formula I is much narrower. Y has to be F and X has to be F, Cl, Br or Me (except Cl is excised in the conditional amendment). By contrast the PCT allowed X and Y each to be any of H, F, Cl, Br, I, OH and Me.
[304] The new class is significantly different and the skilled person would not think that there had been a mere reduction still leaving the same generic class differing only in size.
[305] The problem is significantly exacerbated by the fact that the narrowing does not correspond to the statements of which possibilities are preferred: Y’s having to be F was one of the preferences expressed, but at the same time it was said that X ought also preferably to be F, H or OH, and the proposed amended claim keeps F but discards H and OH and replaces them with F, Cl, Br and Me, a mixture of those preferred and those not said to be preferred. There are also more detailed preferences for X and Y in the PCT depending on the base, but the amended claims do not correspond to them, either.
[306] When EP190 says that there are benefits to the compounds disclosed it is talking about a different class of compounds from those the PCT identified. There was no such claim for the class in the PCT.."
The judge turned to EP365 at [320] and said
"EP365 cannot be any better than EP190 because its choices for X and Y are either the same as the conditionally amended form of EP190 (for claim 1 of EP365) or even narrower (for claim 2 of EP365 where X has to be F or Me, and there is also an addition in the text of EP 365 at [0020] reflecting this)."
"EP365 cannot be any better than EP190 because its choices for X and Y are either the same as the conditionally amended form of EP190 (for claim 1 of EP365) or even narrower (for claim 2 of EP365 where X has to be F or Me, and there is also an addition in the text of EP 365 at [0020] reflecting this)."
It followed that his conclusions on EP190 meant that EP365 was also invalid for added matter.
Lack of Plausibility and Industrial Applicability
Because they were closely related, the learned judge took Gilead's lack of plausibility and industrial applicability together.
Lack of Industrial Applicability
One of the conditions for the grant of a European patent under art 52 (1) EPC is that it should be susceptible of industrial application and s.1 (1) (c) of the Patents Act 1977 is to similar effect. Art 57 of the EPC provides that "an invention shall be considered as susceptible of industrial application if it can be made or used in any kind of industry, including agriculture." S.4 of the Act states that an invention shall be taken to be capable of industrial application if it can be made or used in any kind of industry, including agriculture.
Lord Neuberger had discussed the meaning of the words "industrial applicability" in para [107] of his judgment in Human Genome Sciences Inc v Eli Lilly and Compan [2012] 1 All ER 1154, [2011] UKSC 51, [2012] Bus LR D37, [2012] RPC 6:
"(i) The patent must disclose 'a practical application' and 'some profitable use' for the claimed substance, so that the ensuing monopoly 'can be expected [to lead to] some … commercial benefit' (T 0870/04, para 4, T 0898/05, paras 2 and 4);
(ii) A 'concrete benefit', namely the invention's 'use … in industrial practice' must be 'derivable directly from the description', coupled with common general knowledge (T 0898/05, para 6, T 0604/04, para 15);
(iii) A merely 'speculative' use will not suffice, so 'a vague and speculative indication of possible objectives that might or might not be achievable' will not do (T 0870/04, para 21 and T 0898/05, paras 6 and 21);
(iv) The patent and common general knowledge must enable the skilled person 'to reproduce' or 'exploit' the claimed invention without 'undue burden', or having to carry out 'a research programme' (T 0604/04, para 22, T 0898/05, para 6)."
Lord Neuberger had discussed the meaning of the words "industrial applicability" in para [107] of his judgment in Human Genome Sciences Inc v Eli Lilly and Compan [2012] 1 All ER 1154, [2011] UKSC 51, [2012] Bus LR D37, [2012] RPC 6:
"(i) The patent must disclose 'a practical application' and 'some profitable use' for the claimed substance, so that the ensuing monopoly 'can be expected [to lead to] some … commercial benefit' (T 0870/04, para 4, T 0898/05, paras 2 and 4);
(ii) A 'concrete benefit', namely the invention's 'use … in industrial practice' must be 'derivable directly from the description', coupled with common general knowledge (T 0898/05, para 6, T 0604/04, para 15);
(iii) A merely 'speculative' use will not suffice, so 'a vague and speculative indication of possible objectives that might or might not be achievable' will not do (T 0870/04, para 21 and T 0898/05, paras 6 and 21);
(iv) The patent and common general knowledge must enable the skilled person 'to reproduce' or 'exploit' the claimed invention without 'undue burden', or having to carry out 'a research programme' (T 0604/04, para 22, T 0898/05, para 6)."
It was common ground that the invention must have some practical application though there was some dispute between the parties as to what that might entail,
Plausibility
Mr Justice Meade referred to his own judgment in Sandoz & Teva v. Bristol-Myers Squibb [2022] EWHC 822 and Lord Justice Birss's three-point test at para [53] of his judgment in FibroGen Inc v Akebia Therapeutics Inc [2021] EWCA Civ 1279:
"i) First, what falls within the scope of the claimed class?
ii) Second, what does it mean to say that the invention works?
iii) Third, is it possible to make a reasonable prediction the invention will work with substantially everything falling within the scope of the claim?"
"i) First, what falls within the scope of the claimed class?
ii) Second, what does it mean to say that the invention works?
iii) Third, is it possible to make a reasonable prediction the invention will work with substantially everything falling within the scope of the claim?"
He observed that the Court of Appeal had explained that where claims are to compounds as such, defined by structure (e.g. by means of a Markush formula) the Court has to interpret the specification to identify what utility they are said to have. He also adopted Lord Sumption's formulation of sufficiency from para [37] of his judgment in Warner-Lambert Company LLC v Generics (UK) Ltd (t/a Mylan) and another : (2019) 165 BMLR 14, [2019] Bus LR 360, [2018] UKSC 56, [2019] 3 All ER 95, [2018] RPC 21 “something that would cause the skilled person to think that there was a reasonable prospect that the assertion would prove to be true."
In response to a request by Gilead for further information under CPR Part 18, NuCana alleged that the technical contribution of its invention was a new class of phosphoramidite nucleosides with cytotoxic activity. After considering the expert evidence in support of this allegation, the judge concluded at para [369] that the skilled team would positively think that a significant number of compounds within the claims of the patents would not have either “meaningful” cytotoxic activity or activity at the level of an IC50 of 100µM. The skilled team would also positively expect that a significant number of compounds within the claims of the Patents would have cytotoxic activity in one of those senses. However, the skilled team would be completely unable to predict what or how many compounds would be in either of those two categories.
He reached that conclusion for two reasons:
"i) The fact that the claims cover large numbers of compounds in which multiple changes known to be prone to remove activity are made makes it extremely unlikely that substantially all would have activity at the levels discussed above, so much so that the skilled team would positively think that many would not.
ii) The fact that the claims cover combinations at the 2’ position which could be seen in the CGK such as Watanabe, and inferred from the “sugar pucker” thinking, to be really quite fundamental differences from gemcitabine and liable to remove activity."
The upshot was that the skilled team would not think it plausible that meaningful cytotoxic activity would be preserved across the range of possibilities encompassed by the claims of the patents. They would think that the effect of the substituents in combination was unpredictable. They would not be able to predict how many such non-working combinations there might be, but they would not expect them to be rare one-offs. Since the skilled team would positively think that a substantial number of compounds in the claims would lack activity, it followed that the patents were invalid for implausibility.
In view of his finding on plausibility, his lordship did not consider it necessary to consider lack of industrial applicability. It had been argued in case the lack of plausibility point failed.
Obviousness
Gilead contended that the patents in suit were obvious over US patent application 2003/0109697 A1 for novel phosphoramidite compounds and methods of use that had been invented by H. Michael Shepard and others ("Shepard"). This was not a conventional obviousness but a squeeze between conventional obviousness and plausibility. There was no written expert evidence and the case was argued on the basis of want of technical contribution. The judge did not see how that cause of action could add to the plausibility arguments.
Undue Burden
Art 83 EPC requires a European patent application to disclose the invention in a manner sufficiently clear and complete for it to be carried out by a person skilled in the art. That requirement is not satisfied if the person skilled in the art struggles to perform the invention if he ir she has regard only to the teaching of the patent and his or her common general knowledge. In Mentor v Hollister [1991] FSR 557 Mr Justice Aldous said that that some trial and error is to be expected but it should not amount to reinvention. Anything more amounts to an "undue burden".
Particular issues arise with Markush claims where only some of the compounds within a specified range may have the specified qualities but many more will not. In FibroGen v Akebia the Court of Appeal held in relation to Markush claims that the skilled addressee must be able to identify some compounds beyond those named in the patent without undue burden if the patent is to be valid.
In this case, Gilead alleged that 2MU2FD, the nucleoside component of sofosbuvir, could not be made without undue burden. According to EP190 discloses that certan compounds can be made by reacting a nucleoside derivative with a phosphochloridate. Between para [447] and para [485], the judge explored the "routes" or processes for preparing the compound. He compared the evidence of the parties' experts and the teams that had set out to work the patent.
At para [567] the judge accepted that making the 2MU2FD compounds was intrinsically a difficult task, in absolute terms and in comparison to various otherwise similar nucleoside analogues. He also accepted that the time involved would be considerable and very dependent on chance. He based his finding on the evidence of Gilead's expert but was cautious to remind himself that that expert was really addressing whether he could succeed or not and not whether the task was a routine one for the skilled chemist. His lordship directed himself to weigh the evidence against the legal standard established in cases like Mentor v Hollister with care. He noted in particular, that "just as the fact that some succeeded does not mean that the standard is met, the fact that others failed (completely or initially) does not mean the effort required was undue; the law permits initial failures and recognises that in some fields they are just a fact of life."
He took account of the following factors in para [570]:
"i) Quite a number of routes were put forward by the experts and tried by real workers.
ii) This was not because they were spoilt for choice but because of the nature and difficulty of the task and because it was not a well precedented one. A number of routes were needed as candidates because of the very real probability that each would fail. But it was not predictable which would fail or why.
iii) So this was not a situation where the right general approach was clear but tweaks or fine tuning would be expected if there were initial failures. It would have been a quite a different matter if it was plain, for example, that a particular fluorination approach ought to work and it was just a question of empirically identifying the reaction time and temperature, expecting both that the first try would not work but that a subsequent one in due course would.
iv) The experts could not and did not agree about which routes were preferred, and did not put forward the same possibilities. Again, this was not just a matter of taste or embarras de richesses, but because of the complexity of the task.
v) Each route had its 'pinch points' or areas of greater expected difficulty. Prof Davies did not really recognise these, but Prof Micklefield did.
vi) The pattern of success and failure for the different real teams with different routes was different. There was not a clear winning strategy.
vii) There was a big role for sheer luck.
viii) Whatever one might conclude about the detailed reasons for success or failure with each route by each team, none of the real workers went into the exercise with the expectation that it would be straightforward.
ix) Of the real world workers who tried the task (excluding the CROs) and succeeded, both Mr Clark’s team and Prof Seley-Radtke’s team had their work reported in peer-reviewed journals, and Prof Seley-Radtke’s group reported that the work was “nontrivial” and had produced only low yields (I recognise that the paper also reported much other work). This is a rather prosaic point, perhaps, and it might be said that it is somewhat indirect, but I think it is significant. One would not expect a merely routine synthesis to generate publications like this."
He took account of the following factors in para [570]:
"i) Quite a number of routes were put forward by the experts and tried by real workers.
ii) This was not because they were spoilt for choice but because of the nature and difficulty of the task and because it was not a well precedented one. A number of routes were needed as candidates because of the very real probability that each would fail. But it was not predictable which would fail or why.
iii) So this was not a situation where the right general approach was clear but tweaks or fine tuning would be expected if there were initial failures. It would have been a quite a different matter if it was plain, for example, that a particular fluorination approach ought to work and it was just a question of empirically identifying the reaction time and temperature, expecting both that the first try would not work but that a subsequent one in due course would.
iv) The experts could not and did not agree about which routes were preferred, and did not put forward the same possibilities. Again, this was not just a matter of taste or embarras de richesses, but because of the complexity of the task.
v) Each route had its 'pinch points' or areas of greater expected difficulty. Prof Davies did not really recognise these, but Prof Micklefield did.
vi) The pattern of success and failure for the different real teams with different routes was different. There was not a clear winning strategy.
vii) There was a big role for sheer luck.
viii) Whatever one might conclude about the detailed reasons for success or failure with each route by each team, none of the real workers went into the exercise with the expectation that it would be straightforward.
ix) Of the real world workers who tried the task (excluding the CROs) and succeeded, both Mr Clark’s team and Prof Seley-Radtke’s team had their work reported in peer-reviewed journals, and Prof Seley-Radtke’s group reported that the work was “nontrivial” and had produced only low yields (I recognise that the paper also reported much other work). This is a rather prosaic point, perhaps, and it might be said that it is somewhat indirect, but I think it is significant. One would not expect a merely routine synthesis to generate publications like this."
In the next paragraph, the judge considered the experience of those who had set out to make the compound some of whom had failed while others had succeeded. He concluded "that no person genuinely representative of the ordinary skilled person and unassisted by super-experts and/or hindsight has been shown to have succeeded. I recognise that this cannot be conclusive and is not a substitute for the overall assessment that I have to make but it is important. I think the complete failure by Idenix and the patchy successes and failures of the other workers paints a picture of the task being well beyond the routine and of uncertain result."
It followed that the requirement of art 83 had not been met because the invention could not be performed by a skilled addressee without undue influence.
It followed that the requirement of art 83 had not been met because the invention could not be performed by a skilled addressee without undue influence.
Proposed Amendments
I have already covered this ground. The proposed amendments were refused because they would have extended the protection conferred.
I have already covered this ground. The proposed amendments were refused because they would have extended the protection conferred.
Infringement
I noted above that the judge could see no real defence to the counterclaim. At para [217] he said that sofosbuvir would infringe if the claims of the Patents were valid, but they are not.
Comment
This judgment is a very long and detailed one because it explores the law relating to added matter, plausibility and undue burden insufficiency. It is bound to be cited frequently on all three points of law. Anyone wishing to discuss this case note may call me on 020 7404 5252 during office hours or send me a message through my contact page.


Comments