Patents - Alcon v Actavis
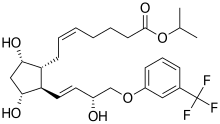 |
| 2D Structure of Travoprost Author Vaccinationist Source Wikimedia Commons |
Patents Court (Mr Justice Meade) Alcon Research LLC and other v Actavis Group PTC EHF and others [2021] EWHC 1026 (Pat) (23 April 2021)
This was an action for relief for the infringement of European patent (UK) 1 920 764 which had expired in 2014 and a counterclaim for its revocation on grounds of anticipation, obviousness and insufficiency. The defendants admitted that their products would have infringed the patent had it been valid so these were effectively revocation proceedings. A supplementary protection certificate numbered SPC/GB12/038 had been granted for Travoprost upon the expiry of the patent but the only issue that related to the SPC depended on the validity of the patent. The action and counterclaim came on for trial before Mr Justice Meade between 16 and 22 March 2021 and his lordship delivered judgment on 23 April 2021 (see Alcon Research LLC and other v Actavis Group PTC EHF and others [2021] EWHC 1026 (Pat) (23 April 2021)). At para [202] of his judgment, Mr Justice Meade found the patent to have been valid. No new law is to be found in this judgment but the judgment is well worth reading for some useful commentary on the applicable law.
What is an Invention?
This question is answered by s.125 (1) of the Patents Act 1977:
"For the purposes of this Act an invention for a patent for which an application has been made or for which a patent has been granted shall, unless the context otherwise requires, be taken to be that specified in a claim of the specification of the application or patent, as the case may be, as interpreted by the description and any drawings contained in that specification, and the extent of the protection conferred by a patent or application for a patent shall be determined accordingly."
"For the purposes of this Act an invention for a patent for which an application has been made or for which a patent has been granted shall, unless the context otherwise requires, be taken to be that specified in a claim of the specification of the application or patent, as the case may be, as interpreted by the description and any drawings contained in that specification, and the extent of the protection conferred by a patent or application for a patent shall be determined accordingly."
What is meant by the "Claims"?
A "claim" is part of the specification of a patent. S.14 (2) (b) of the Act requires every patent application to contain inter alia "a specification containing a description of the invention, a claim or claims and any drawing referred to in the description or any claim." This subsection summarizes the bargain between public and inventor. In consideration of teaching the public how to make or use the invention the public grants to the inventor the monopoly ser out in the claims". S.14 (5) requires the claim or claims to:
"(a) define the matter for which the applicant seeks protection;
(b) be clear and concise;
(c) be supported by the description; and
(d) relate to one invention or to a group of inventions which are so linked as to form a single inventive concept."
Which Claims were in Dispute?
"(a) define the matter for which the applicant seeks protection;
(b) be clear and concise;
(c) be supported by the description; and
(d) relate to one invention or to a group of inventions which are so linked as to form a single inventive concept."
Which Claims were in Dispute?
The defendants challenged claims 1 and 2. These were considered by the judge between paras [113] and [[119]. Were the novelty of either of those claims successfully challenged, the claimants would have applied for their amendment.
Anticipation
One of the conditions for the grant of a patent is that the invention is new (see s.1 (1) (a) of the Patents Act 1977). S,2 (1) of the Act provides that
The next 2 subsections of that section define the "state of the art". That does not have to be considered at this stage. If an invention is not new, it is said to be "anticipated". At para [129] of his judgment, Mr Justice Meade said that the basic test of anticipation is whether there has been a clear and unambiguous disclosure of all the features of a claim.
The prior art relied upon by the defendants was European patent application EP0603800 for Prostaglandin combinations in glaucoma therapy. The features relied on were not to be found in any of the claims but in the general teaching of the application. The challenge failed because EP0603800 did not disclose clearly and unambiguously all the features of the patented invention. As the judge said at [136]:
The prior art relied upon by the defendants was European patent application EP0603800 for Prostaglandin combinations in glaucoma therapy. The features relied on were not to be found in any of the claims but in the general teaching of the application. The challenge failed because EP0603800 did not disclose clearly and unambiguously all the features of the patented invention. As the judge said at [136]:
Other objections were that there was no plausibility for what was said to have been disclosed in the prior invention and that the prior art had not anticipated the dosage range in the patent in suit.
Obviousness
Another condition for the grant of a patent is that the invention must involve an inventive step (s.1 (1) (b) of the Patents Act 1977). S.3 provides:
"An invention shall be taken to involve an inventive step if it is not obvious to a person skilled in the art, having regard to any matter which forms part of the state of the art by virtue only of section 2 (2) above (and disregarding section 2 (3) above)."
"An invention shall be taken to involve an inventive step if it is not obvious to a person skilled in the art, having regard to any matter which forms part of the state of the art by virtue only of section 2 (2) above (and disregarding section 2 (3) above)."
This section was considered by the Supreme Court in Actavis Group PTC EHF and others v ICOS Corporation and another [2019] UKSC 15 (27 March 2019) [2019] Bus LR 1318 between paras [52] and [73]. Lord Hodge, who delivered the judgment of the Court, noted at [60]:
'(1) (a) Identify the notional "person skilled in the art";
(b) Identify the relevant common general knowledge of that person;
(2) Identify the inventive concept of the claim in question or if that cannot readily be done, construe it;
(3) Identify what, if any, differences exist between the matter cited as forming part of the "state of the art" and the inventive concept of the claim or the claim as construed;
(4) Viewed without any knowledge of the alleged invention as claimed, do those differences constitute steps which would have been obvious to the person skilled in the art or do they require any degree of invention?'
(Pozzoli SPA v BDMO SA [2007] EWCA Civ 588; [2007] FSR 37, para 23 per Jacob LJ),"
"Person Skilled in the Art!
In identifying the "person skilled in the art" Mr Justice Meade said at para [30]:
"The parties cited several authorities on the correct approach to this issue, but in my view, there was nothing they referred to (apart from the EPO point mentioned below) that was not taken into account in the very detailed review by Birss LJ, in one of his last decisions at first instance, Illumina Cambridge Ltd. v. Latvia MGI Tech SIA and others [2021] EWHC 57 (Pat)."
"The parties cited several authorities on the correct approach to this issue, but in my view, there was nothing they referred to (apart from the EPO point mentioned below) that was not taken into account in the very detailed review by Birss LJ, in one of his last decisions at first instance, Illumina Cambridge Ltd. v. Latvia MGI Tech SIA and others [2021] EWHC 57 (Pat)."
Mr Justice Meade quoted paras [56] to [70] pf Mr Justice Birss's judgment in that case and added at para [31] of his own judgment:
"i) The requirements not to be unfair to the patentee by allowing an artificially narrow definition, or unfair to the public (and the defendant) by going so broad as to “dilute” the CGK. Thus, as Counsel for Alcon accepted, there is an element of value judgment in the assessment.
ii) The fact that I must consider the real situation at the priority date, and in particular what teams existed.
iii) The need to look for an “established field”, which might be a research field or a field of manufacture.
iv) The starting point is the identification of the problem that the invention aims to solve."
ii) The fact that I must consider the real situation at the priority date, and in particular what teams existed.
iii) The need to look for an “established field”, which might be a research field or a field of manufacture.
iv) The starting point is the identification of the problem that the invention aims to solve."
In this context, it may assist the reader to read my case note Patents: Illumina Cambridge v MGI Tech of 5 Feb 2021 and in particular the paragraph headed "The Identity of the Skilled Team". The judge set out the applicable facts between paras [33] and [42] before concluding that the skilled addressee, in this case, would be a team that would include a prostaglandin specialist.
Common General Knowledge
The reference to the "state of the art" in s.3 of the Act is confusing because it would be fanciful to suppose that any skilled addressee in any field would ever know more than a fraction of the
"matter (whether a product, a process, information about either, or anything else) which has at any time before the priority date of that invention been made available to the public (whether in the United Kingdom or elsewhere) by written or oral description, by use or in any other way."
All that could reasonably be expected is that "persons skilled in the art" (that is to say, experts in the field would share various tenets. Expert witnesses state what they believe those tenets to be in their reports and oral evidence. Where there is a dispute the judge has to decide after taking the account the testing of their evidence by cross-examination and the arguments of counsel.
In this case, the judge directed the parties to identify the agreed common general knowledge between paras [44] and [71] and the disputed common general knowledge between [72] and [77]. He made his findings on what constituted common general knowledge between para [78] and para [98].
Inventive Concept
According to the claimant. the technical contribution of the patent was:
"i) A new and useful therapy for the treatment of glaucoma (of course this is on the assumption that the anticipation attack failed);
ii) A prostaglandin analogue which lowers IOP but without the hyperemia associated with PGF2a;
iii) A prostaglandin analogue which is better than latanoprost in terms of IOP lowering but with comparable good/acceptable hyperemic properties;
iv) A prostaglandin analogue with less hyperemia than the structurally closest analogue from the Stjernschantz 417 patent application identified in the Patent."
The judge did not see the relevance of point (iv) but he accepted the other three.
"i) A new and useful therapy for the treatment of glaucoma (of course this is on the assumption that the anticipation attack failed);
ii) A prostaglandin analogue which lowers IOP but without the hyperemia associated with PGF2a;
iii) A prostaglandin analogue which is better than latanoprost in terms of IOP lowering but with comparable good/acceptable hyperemic properties;
iv) A prostaglandin analogue with less hyperemia than the structurally closest analogue from the Stjernschantz 417 patent application identified in the Patent."
The judge did not see the relevance of point (iv) but he accepted the other three.
Differences between the "State of the Art" and the Inventive Concept
The defendants relied on an article by Stjernschantz and Resul entitled "Phenyl substituted prostaglandin analogs for glaucoma” which was published in Drugs of the Future, 1992 Vol 17(8): 691-704 published in October 1992. The judge summarized that article between para [142] and [164]. At para [168] he considered the claimants' contention on the differences between the article and the inventive concept:
"Alcon characterised there as being three differences, by reference to the structures of latanoprost (which it says was the most promising of the compounds in Stjernschantz) and of FIE, as follows:"
At [169] he considered the defendants' case that the difference was merely the choice of a different selective FP receptor agonist, i.e. FIE. The judge observed at [170] that:
"This was more than merely a presentational issue of the patentee seeking to maximise the number of differences and the defendant trying to minimise it. The disparity in the way the parties characterised the differences was more substantive than that and related to the nature of the skilled team and the way they would see the work reported in Stjernschantz. Alcon’s position had a much greater focus on the medicinal chemistry content of Stjernschantz and its SAR work, and that is why it described the differences in terms of structure. I return to this below. My conclusion is that Alcon’s approach better reflects the contents and approach of Stjernschantz and the presence of a medicinal chemist as an active member of the skilled team."
Whether hose Differences constitute Steps which would have been obvious to the Person skilled in the Art or do they require any Degree of Invention?'
"Alcon characterised there as being three differences, by reference to the structures of latanoprost (which it says was the most promising of the compounds in Stjernschantz) and of FIE, as follows:"

At [169] he considered the defendants' case that the difference was merely the choice of a different selective FP receptor agonist, i.e. FIE. The judge observed at [170] that:
"This was more than merely a presentational issue of the patentee seeking to maximise the number of differences and the defendant trying to minimise it. The disparity in the way the parties characterised the differences was more substantive than that and related to the nature of the skilled team and the way they would see the work reported in Stjernschantz. Alcon’s position had a much greater focus on the medicinal chemistry content of Stjernschantz and its SAR work, and that is why it described the differences in terms of structure. I return to this below. My conclusion is that Alcon’s approach better reflects the contents and approach of Stjernschantz and the presence of a medicinal chemist as an active member of the skilled team."
Mr Justice Meade said at [171] that "the task for the Defendants was to show that it was obvious to use FIE, instead of any of the analogues in Stjernschantz, to treat glaucoma.". After considering their arguments between para [172] and para [188] and their evidence para [189] to [195] he concluded that the differences would not have been obvious and that they would have required a degree of invention.
Insufficiency
S.14 (3) of the Patents Act 1977 requires the specification of an application to disclose the invention in a manner which is clear enough and complete enough for the invention to be performed by a person skilled in the art. If it fails to do so the patent may be revoked under s.72 (1) (c) of the Patents Act 1977. Sometimes it is possible to argue that if a specification is construed one way then it is obvious; but, if it is construed another way, it will not disclose the invention in a manner that is clear or complete enough for it to be performed. That is known as a "squeeze",
The defendants alleged a squeeze in this case:
“3. In the event that the claims of the Patent are not obvious because it was understood that the administration of PGF2a isopropyl ester would cause ocular inflammation and irritation, the Patent fails to make plausible that the compounds of the invention are suitable for use in treating glaucoma and ocular hypertension. In particular, the data in the specification of the Patent fails to evidence an improvement in these side effects when the compounds of the invention are used over PGF2a isopropyl ester.”
“3. In the event that the claims of the Patent are not obvious because it was understood that the administration of PGF2a isopropyl ester would cause ocular inflammation and irritation, the Patent fails to make plausible that the compounds of the invention are suitable for use in treating glaucoma and ocular hypertension. In particular, the data in the specification of the Patent fails to evidence an improvement in these side effects when the compounds of the invention are used over PGF2a isopropyl ester.”
The learned judge had no trouble in disposing of the argument:
"[199] The point fails on the facts, however, since, as I have explained above, the Patent does make it plausible that FIE causes reduced hyperemia compared to PGF2a.
200. The pleading does not allege that the Patent does not make plausible a better effect on IOP lowering. I have mentioned this above, and that the Defendants did not press the argument.
201. Overall, therefore, the squeeze made no difference and does not help the Defendants."
"[199] The point fails on the facts, however, since, as I have explained above, the Patent does make it plausible that FIE causes reduced hyperemia compared to PGF2a.
200. The pleading does not allege that the Patent does not make plausible a better effect on IOP lowering. I have mentioned this above, and that the Defendants did not press the argument.
201. Overall, therefore, the squeeze made no difference and does not help the Defendants."
Conclusion
As the anticipation, obviousness and insufficiency attacks failed and as infringement had already been admitted, the patent was valid and infringed.
Anyone wishing to discuss this case or patents generally should call me on 020 7404 5252 during office hours or send me a message through my contact form.
Anyone wishing to discuss this case or patents generally should call me on 020 7404 5252 during office hours or send me a message through my contact form.


Comments